No Media
Abstract
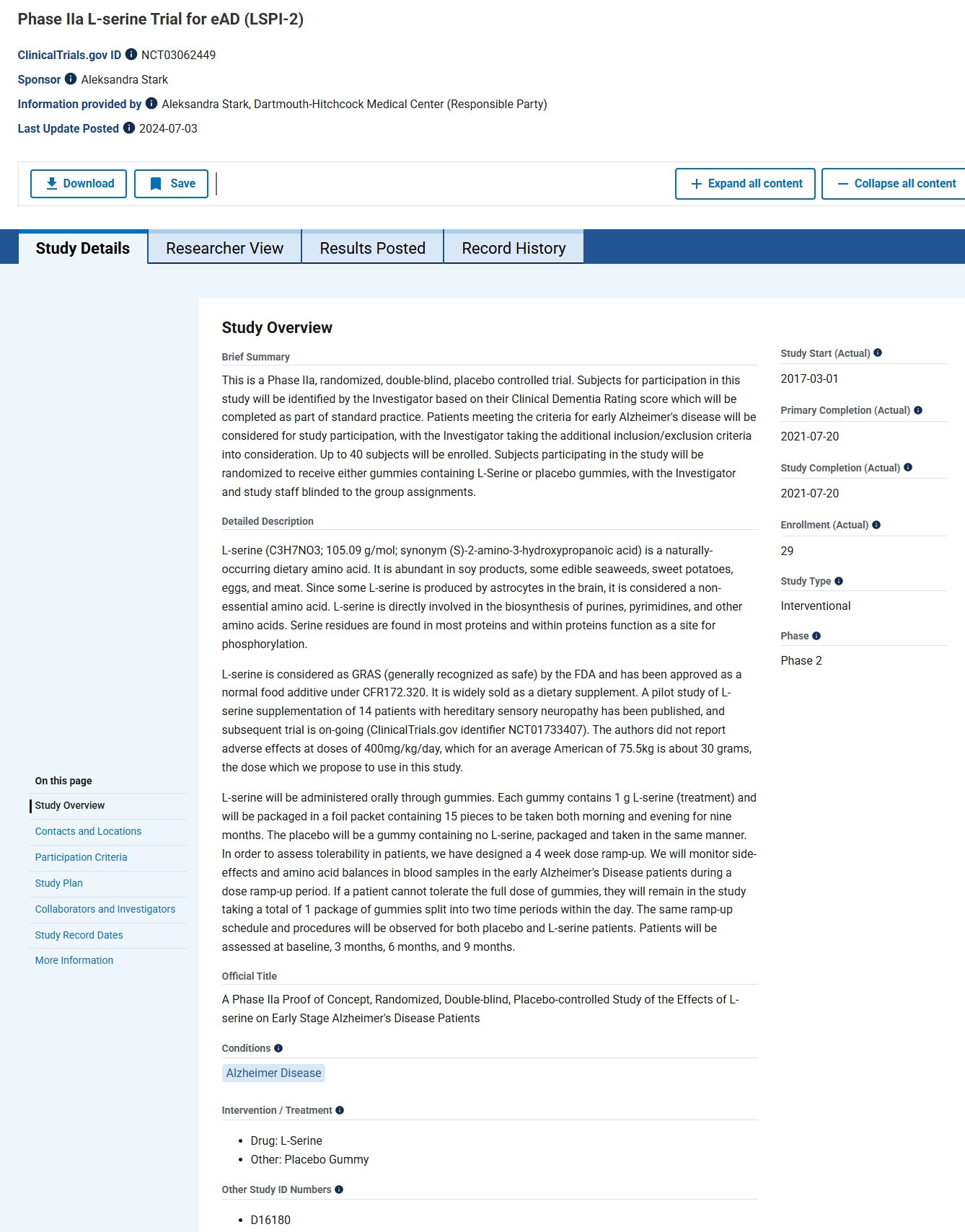
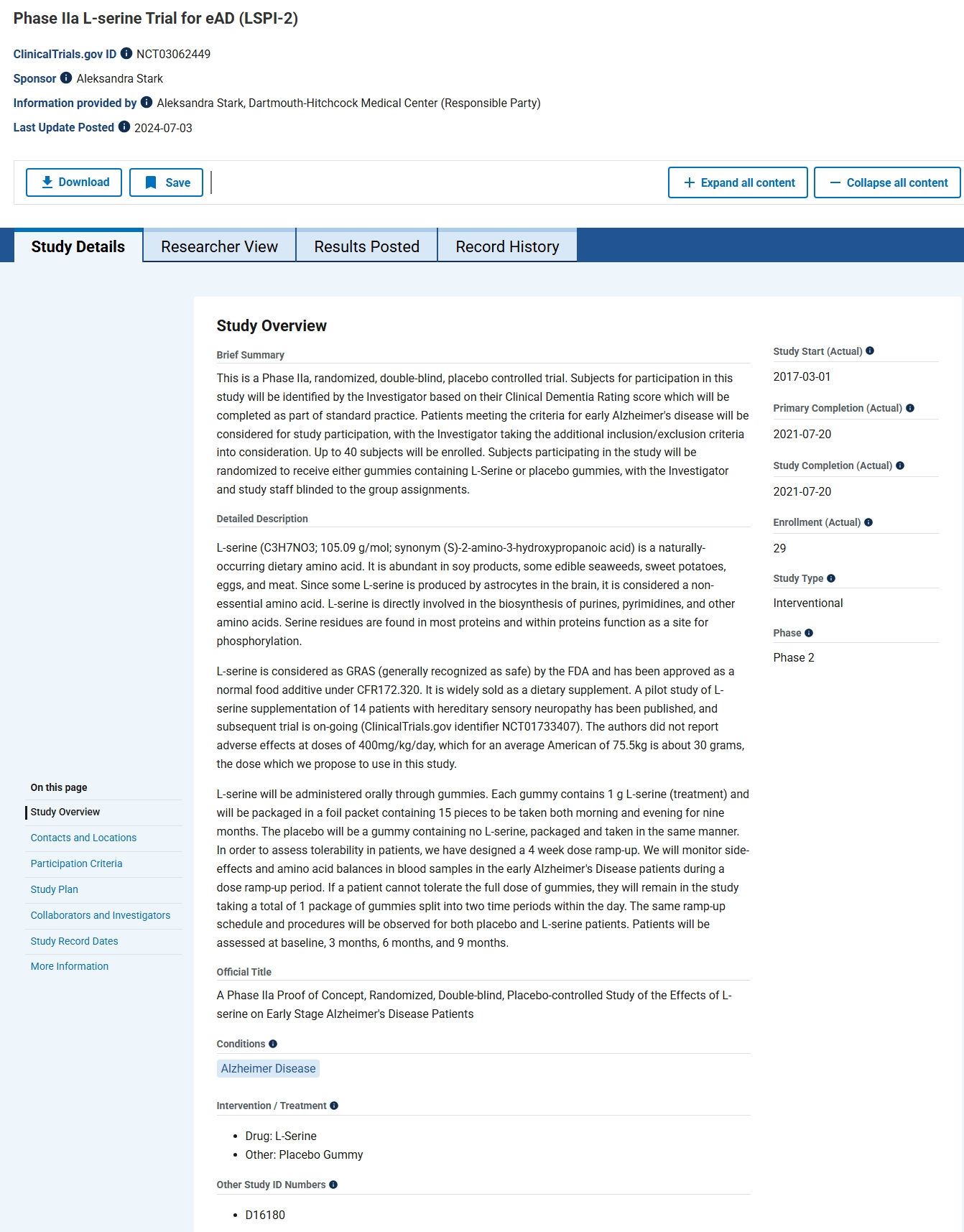
This systematic review analyzes monosodium glutamate (MSG) in the Alzheimer’s disease-like condition to enhance translational research. Our review seeks to understand how MSG affects the brain and causes degenerative disorders. Due to significant preclinical data linking glutamate toxicity to Alzheimer’s disease and the lack of a comprehensive review or meta-analysis, we initiated a study on MSG’s potential link. We searched PubMed, ScienceDirect, ProQuest, DOAJ, and Scopus for animal research and English language papers without time constraints. This study used the PRISMA-P framework and PICO technique to collect population, intervention or exposure, comparison, and result data. It was registered in PROSPERO as CRD42022371502. MSG affected mice’s exploratory behaviors and short-term working memory. The brain, hippocampus, and cerebellar tissue demonstrated neuronal injury-related histological and histomorphometric changes. A total of 70% of MSG-treated mice had poor nesting behavior. The treated mice also had more hyperphosphorylated tau protein in their cortical and hippocampus neurons. Glutamate and glutamine levels in the brain increased with MSG, and dose-dependent mixed horizontal locomotor, grooming, and anxiety responses reduced. MSG treatment significantly decreased phospho-CREB protein levels, supporting the idea that neurons were harmed, despite the increased CREB mRNA expression. High MSG doses drastically lower brain tissue and serum serotonin levels. In conclusion, MSG showed AD-like pathology, neuronal atrophy, and short-term memory impairment. Further research with a longer time span and deeper behavioral characterization is needed.
This systematic review analyzes monosodium glutamate (MSG) in the Alzheimer’s disease-like condition to enhance translational research. Our review seeks to understand how MSG affects the brain and causes degenerative disorders. Due to significant preclinical data linking glutamate toxicity to Alzheimer’s disease and the lack of a comprehensive review or meta-analysis, we initiated a study on MSG’s potential link. We searched PubMed, ScienceDirect, ProQuest, DOAJ, and Scopus for animal research and English language papers without time constraints. This study used the PRISMA-P framework and PICO technique to collect population, intervention or exposure, comparison, and result data. It was registered in PROSPERO as CRD42022371502. MSG affected mice’s exploratory behaviors and short-term working memory. The brain, hippocampus, and cerebellar tissue demonstrated neuronal injury-related histological and histomorphometric changes. A total of 70% of MSG-treated mice had poor nesting behavior. The treated mice also had more hyperphosphorylated tau protein in their cortical and hippocampus neurons. Glutamate and glutamine levels in the brain increased with MSG, and dose-dependent mixed horizontal locomotor, grooming, and anxiety responses reduced. MSG treatment significantly decreased phospho-CREB protein levels, supporting the idea that neurons were harmed, despite the increased CREB mRNA expression. High MSG doses drastically lower brain tissue and serum serotonin levels. In conclusion, MSG showed AD-like pathology, neuronal atrophy, and short-term memory impairment. Further research with a longer time span and deeper behavioral characterization is needed.